Integrating more innovative approaches that better ensure human participant protection & data quality
We build quality into clinical research from start to end which supports a dynamic, flexible, and proactive approach to study management and conduct. Quality-by-design (QbD) and risk management are key principles of our Quality Management System framework.
To keep pace with the increasing scale, complexity, and costs of clinical trials and to ensure appropriate use of technology, our team aims to integrate more innovative approaches that better ensure human participant protection and data quality. We strive to enhance the alignment of Quality with Innovation by developing, piloting, and implementing new methods and quality improvements across studies.
Read more about our approaches below.
-
The 3-3-3 approach to Risk Assessment and Risk Management (RARM) is a novel paradigm spearheaded by the Center's Quality Team and subsequently adopted by the WCG Avoca Quality Consortium. When working with research investigators, we utilize the 3-3-3 approach to identify and manage key study risks (see image below). This, in turn, focuses effort on the things that matter most in research - ensuring human participant protection and reliability of study results.
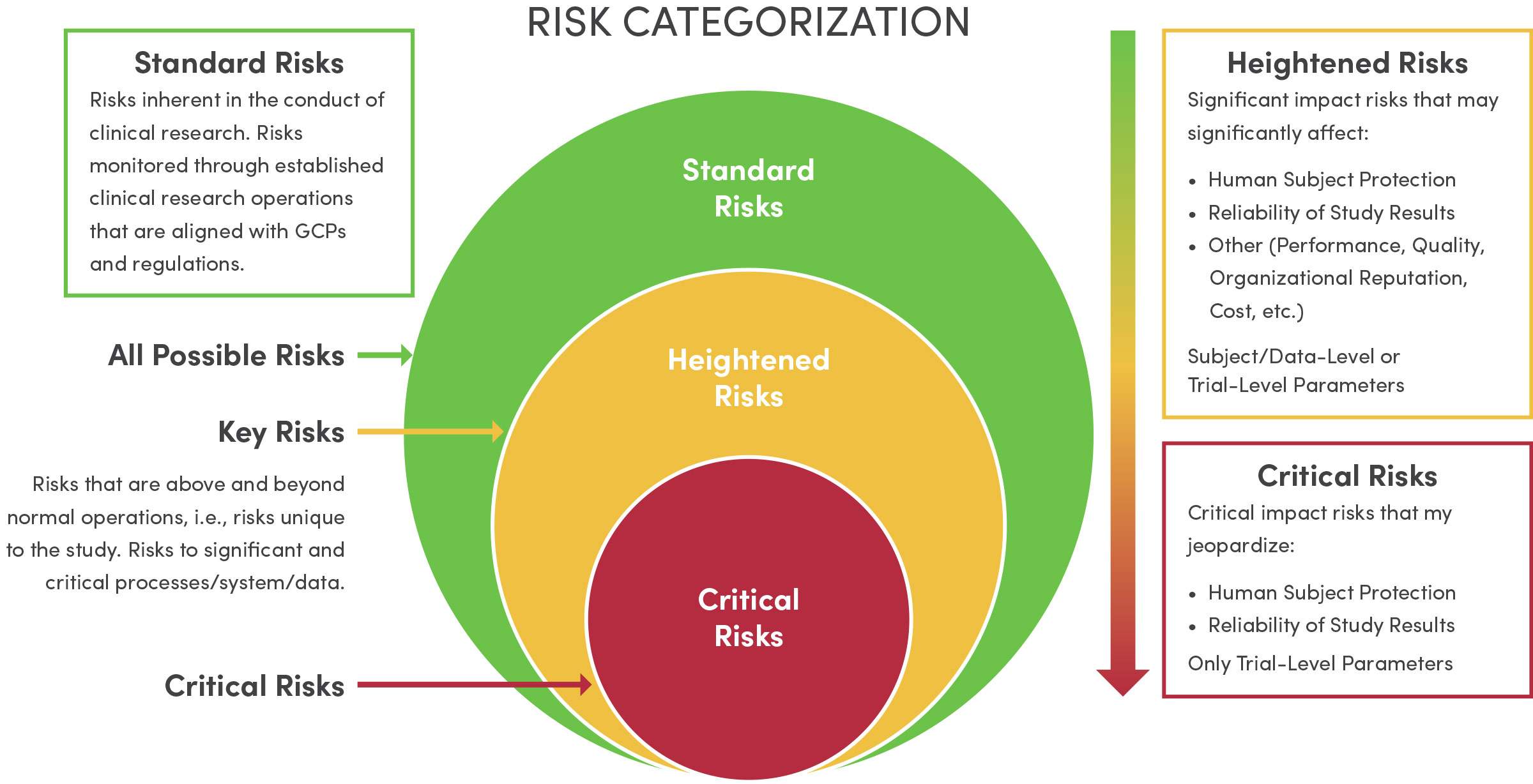
-
Monitoring is a critical aspect of study oversight that contributes to public assurance of human participant protection and reliability of study results. Industry guidelines, regulations, and policies are shifting away from one-size-fits-all approaches and moving towards those that are more risk-based.
We work with research investigators to customize a fit-for-purpose plan to monitor data and manage risks that promote a more dynamic and proactive approach to continuous improvement in study conduct and oversight. Our team utilizes a risk-based strategy that combines varied approaches to monitoring clinical investigations. Efficiencies are gained by focusing source data monitoring on critical data and processes and relying on central and statistical monitoring to signal when additional source data monitoring is warranted. Each study uses a unique combination of source, central, and statistical monitoring that is proportionate to its risks to human participant protection and data reliability.
-
ICH GCP encourages implementation of improved, more efficient approaches to clinical trial oversight. Another method we apply to studies as part of the monitoring strategy is to utilize a risk-based approach to managing protocol deviations.
This approach encourages more efficient oversight that focuses effort on only those issues with a significant or potentially significant impact to human participant protection and reliability of study results.

